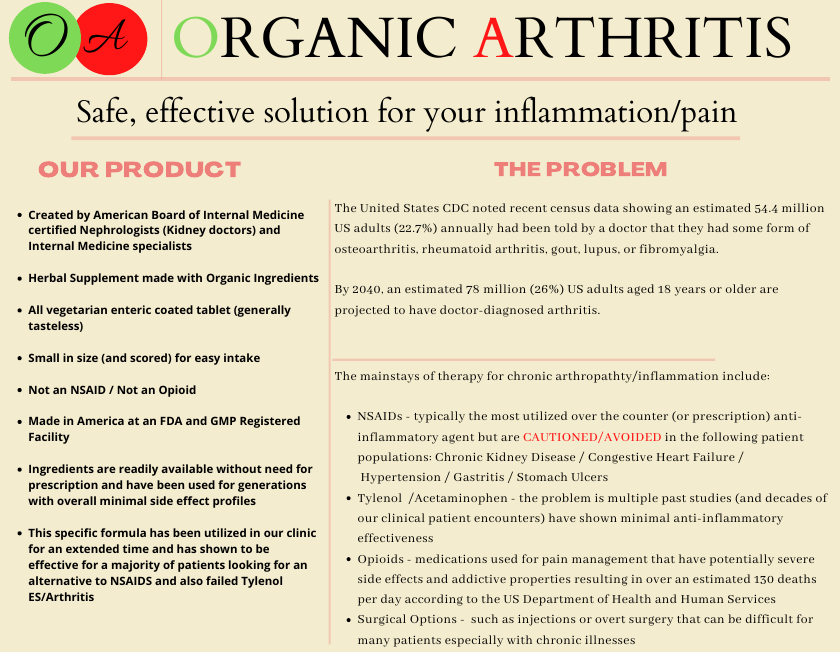
Organic Arthritis - Customer Feedback
Organic Arthritis - Customer Feedback
I can not count the number of times our practice had a patient who was told to avoid NSAIDS who then came to the office with suboptimal relief on Tylenol/Acetaminophen. Came with issues walking/exercising. Issues in the quality of their lives. I generally do not prescribe opioids - especially in the chronic kidney disease population which impairs the body's ability to clear many opioids and can increase the risk of overdose/toxicity. I had to ask them to seek orthopedic specialists to help control their symptoms - but procedural intervention can often times be inconvenient and difficult particularly in patients with multiple medical issues.
We began looking into herbal/holistic substances that have been utilized for generations in inflammatory relief across the world. We parsed basic science/bench as well as clinical research databases. Our ingredients have shown COX or IL37 suppression or urine prostaglandin decrease indicating effectiveness in suppressing the inflammatory cascade with the caveat of no known issues with stomach lining or kidney function / Sodium retention / Blood Pressure elevation.
Study data on one of our key ingredients exhibited a dose-dependent anti-inflammatory effect in bench data comparable to the reference drug Indomethacin ( a very potent prescription strength NSAID) (Mutabagani A - Pharm J 1997; 5: 110-113). Another study on one of our key ingredients noted significant decrease in induced proinflammatory cytokines such as interleukin-1beta (IL-1β), tumor necrosis factor-alpha (TNF-α), metalloproteinase-13 (MMP-13), COX-2, and prostaglandin E2 in an in vitro model (Vaillancourt et al - J Cell Biochem 2011; 112: 107-117).
Also in a multi database meta analysis including several human randomized controlled trials (RCTs) - one of our key ingredients showed marked reduction in documented pain visual analogue score (PVAS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores vs placebo (P < .00001) and there was no significant mean difference in PVAS between our ingredient and pain medicine (typically NSAIDs) in a meta-analysis of five studies (Daily J et al - J Med Food. 2016;19(8):717-729)
In 2014 after some testing and adjustments - we solidified a powder based regimen and would at times suggest patients seeking alternative/additional relief try it. We did not commercially sell it but would just suggest one could obtain and try the ingredients on their own. The response was tremendous and more often than not this became their go to relief agent. The only consistent suggestion (and complaint) was the taste of the powders and the need for a tablet to ease delivery/use.
We went to work and adjusted the extract ratios/concentrations and were able to manufacture (made in the USA at an FDA registered and GMP certified facility) a coated (should be tasteless) tablet that can be scored in half to help ease of use. We launched Organic Arthritis.
We have been blessed to have tremendous support and the feedback to date has been stellar both in our clinic and online with essentially every review coming back at 5 stars. We wanted to gather more concrete data in an observational study format which led us to conducting an observational analysis of patients who were clinically recommended to stop NSAIDS + did not have adequate relief on Acetaminophen who then began using Organic Arthritis with survey follow up at 30 and 60 days. Over 90% of patients surveyed (28/30) noted a degree of efficacious relief of inflammatory symptoms utilizing only Organic Arthritis at recommended dosages. Over 90% of patients reordered our product on their own accord to continue use after the study period ended! This was truly an amazing response and we sincerely hope that our product can safely help many in need of joint/muscle relief!